Research
Our studies focus on understanding the molecular biology of the progression of acute leukemias with erythroid differentiation and leukemias arising from antecedent myeloproliferative neoplasms. Particularly, we are interested in dissecting the regulation of JAK2/STAT pathway and the implication of STAT-regulated inflammatory pathways in the erythroid leukemogenesis. We also investigate novel combinational approaches targeting cytokine signaling, DNA methyltransferases and other oncogenic pathways in erythroid leukemias. We finally study the activity of targeted therapies such RAS inhibitors in acute leukemias arising from antecedent myeloid neoplasms.
Our work incorporates signal transduction studies, CRISPR-Cas9 gene editing via viral transduction and electroporation, CRISPR-Cas9 screens, single cell transcriptomic analysis, and cell line and patient-derived xenograft models.
Research Focus
The focus of the Karantanos lab is the elucidation of the role of inflammatory signaling in the progression of high-risk myeloid neoplasms and the development of novel targeted therapies to improve the outcomes of patients with these diseases. Specifically, we investigate the regulation and functional implication of JAK2/STAT1 signaling in TP53-mutated myeloid neoplasms by incorporating single cell analysis, CRISPR-Cas9 screens, and signal transduction studies. We also develop antibody-based therapies that target inflammatory mediators in TP53-mutated myelodysplastic syndrome/acute myeloid leukemia (MDS/AML) utilizing xenografts and transgenic mouse models. Finally, we study the effect of RAS inhibition in high-risk MDS and chronic myelomonocytic leukemia.
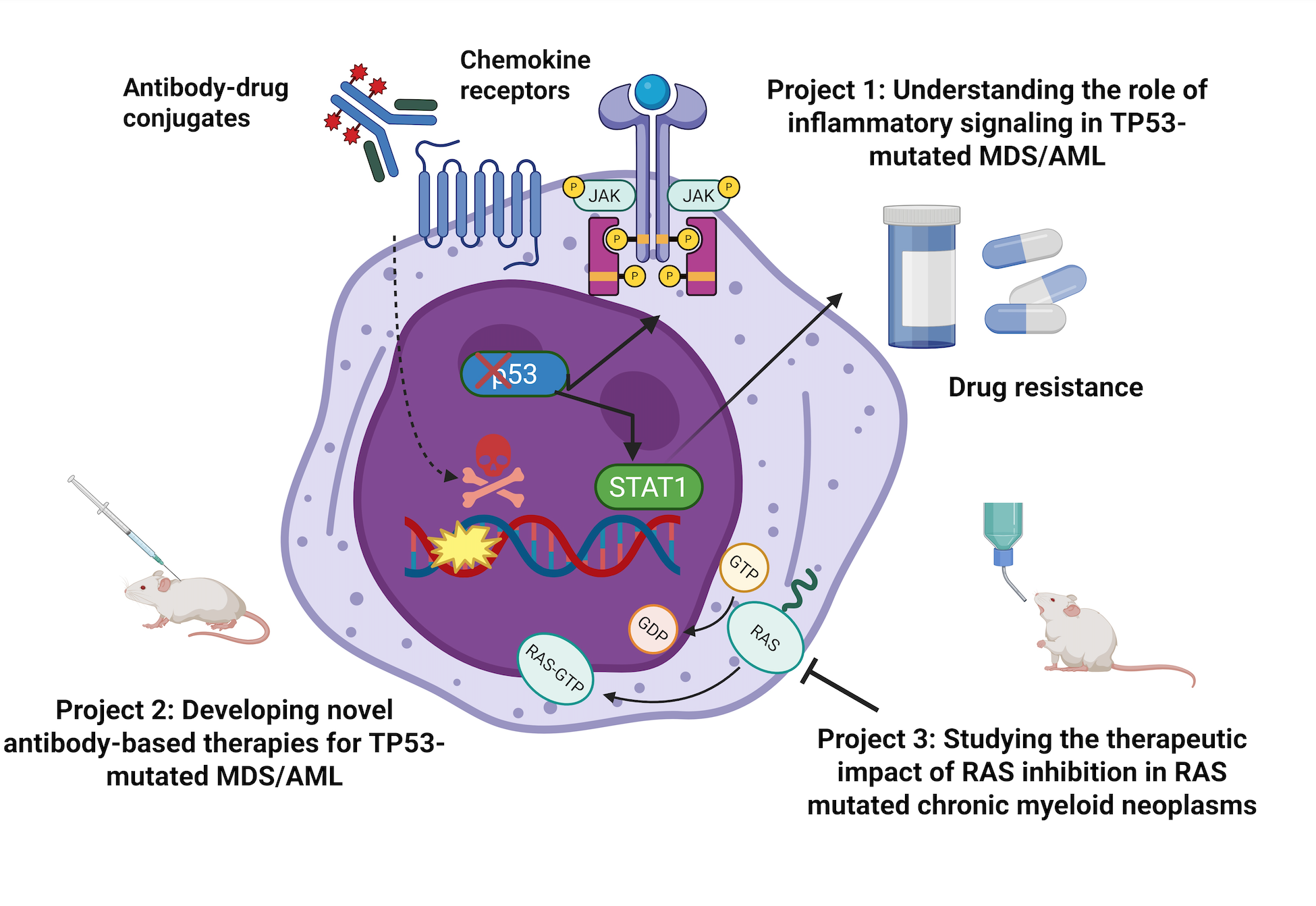
Project 1: Investigation of IFN-γ signaling regulation and functional impact of STAT1 in TP53-mutated MDS/AML
We hypothesize that TP53 deletion induces directly IFN-γ signaling via STAT1 activation and STAT1 is involved in the acquisition of a treatment-resistance phenotype. We will study this hypothesis by incorporating CRISPR-Cas9 gene editing, signal transduction studies, and competitive mouse transplantation experiments.
Project 2: Development of antibody-based therapies for TP53-mutated MDS/AML
We have developed various antibody-drug conjugates targeting inflammatory mediators in the surface of TP53-mutated MDS/AML cells and we are testing them in cell line-, patient-derived xenografts and transgenic mice with TP53 loss.
Project 3: Testing the effect of RAS inhibition in RAS mutated myeloid neoplasms
We are testing the efficacy of RAS inhibition in RAS mutated MDS, CMML and AML arising from MDS/MPN/CMML.
